XIE/Enzyme:2025/Dec: Difference between revisions
(→12.27) |
(→12.27) |
||
| Line 234: | Line 234: | ||
| 6 || 3 || 3 || 3 || 3 || 0 || 0 || 0 | | 6 || 3 || 3 || 3 || 3 || 0 || 0 || 0 | ||
|} | |} | ||
*琼脂糖凝胶电泳 | |||
[[File:Origami_Enzyme_Success.png|thumb|center|center|600px]] | [[File:Origami_Enzyme_Success.png|thumb|center|center|600px]] | ||
Revision as of 13:40, 28 December 2025
12.08
- HR-DNA Origami(NO) Folding
- Materials
| Material | Volume(100 μL) |
|---|---|
| Staples (500 nM) | 25 μL |
| P8064 (150 nM) | 10 μL |
| 10X PBS | 10 μL |
| H2O | 55 μL |
- Program folding in PCR thermocycler : The mixed sample was put on a thermal ramp starting with a rapid heat denaturation at 80 °C for 5 min followed by cooling from 80 °C to 60 °C over 20 min, then slow cooling from 60 °C to 24 °C over 14 h.
- Purification (12.09): Reserve a 10 µL aliquot. Add the remaining 90 µL to a 100‑kDa MWCO centrifugal filter unit and dilute to 500 µL with 1× PBS. Centrifuge at 8,000 g for 1.5 min and discard the filtrate . Add UP Water to 500 ul to the filter unit and repeat the centrifugation; perform this wash step three times. After the final wash, adjust the volume in the filter unit to 100 µL with UP Water, invert the filter unit into a fresh collection tube, and centrifuge at 8,000 g for 1.5 min to collect the concentrate.
12.09
- HR-DNA Origami(NO) Folding
- Materials
| Material | Volume(100 μL) |
|---|---|
| Staples (490 nM) | 25 μL |
| P8064 (150 nM) | 10 μL |
| 10X PBS | 10 μL |
| H2O | 55 μL |
- Program folding in PCR thermocycler : The mixed sample was put on a thermal ramp starting with a rapid heat denaturation at 80 °C for 5 min followed by cooling from 80 °C to 60 °C over 20 min, then slow cooling from 60 °C to 24 °C over 14 h.
- Purification (12.10): Reserve a 10 µL aliquot. Add the remaining 90 µL to a 100‑kDa MWCO centrifugal filter unit and dilute to 500 µL with 1× PBS. Centrifuge at 8,000 g for 1.5 min and discard the filtrate . Add 1X PBS to 500 ul to the filter unit and repeat the centrifugation; perform this wash step three times. After the final wash, adjust the volume in the filter unit to 100 µL with 1X PBS, invert the filter unit into a fresh collection tube, and centrifuge at 8,000 g for 1.5 min to collect the concentrate.
12.10
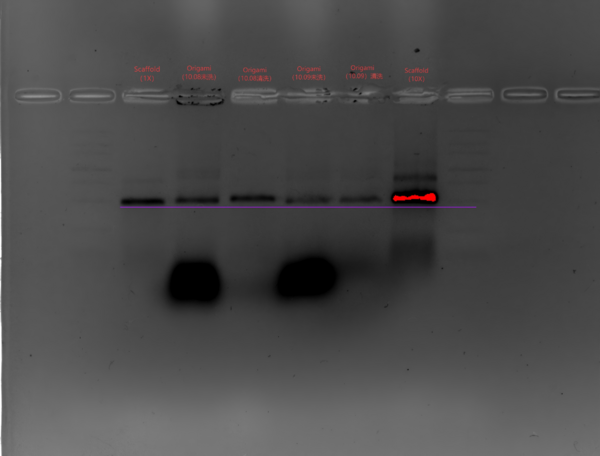
- Agarose Gel Electrophoresis
- Gel Preparation
- Recipe: 2.4 g agarose in 120 mL 0.5× TBE
- Heat in a microwave to dissolve, cool slightly, adjust volume with 0.5× TBE to 120 ul, then add 1.2 mL of 1 M MgCl2 and 12 µL GoldView DNA stain.
- Insert comb and waite for the gel to cool and solidify.
- Sample preparation
- Origami (15 nM): sample:loading buffer = 5:1.
- Scaffold: take 1 µL scaffold, dilute to 10 µL (final concentration 15 nM); scaffold:loading buffer = 5:1.
- Electrophoresis
- Run at 90 V for 120 min in 0.5× TBE with 10 mM MgCl2
- Marker、Scaffold(1X)、Origami(10.08-未洗)、Origami(10.08—清洗)、Origami(10.09-未洗)、Origami(10.09-清洗)、Scaffold(10X)、Marker
12.11
- TEM
Negative staining (uranyl acetate)
- Grid activation: glow‑discharge copper grids for 30 s.
- Sample staining
- apply 4.5 µL of sample onto the grid.
- Blot off the sample, add buffer to the grid, then blot off the buffer.
- Prepare parafilm and place three drops of uranyl acetate (pH adjusted) on the parafilm.
- Quickly transfer the grid into a staining droplet, blot off excess, and incubate for 30 s.
- Air‑dry the grid.
12.12
- S2 conjugated G5
Stock Solution: 1 mM
Incubation
- Measure G5 stock (3 mg/mL, 46 μM). Dilute 10 μL of the stock to 100 μL (4.6 μM); aliquot and store at -80°C as the storage sample.
- Take one storage aliquot and add 10 μL DBCO stock (10 mM). Divide evenly into two groups (ultrafiltration and non-ultrafiltration). For the non-ultrafiltration group, aliquot 16 μL into a PCR tube (designated M1) and incubate on ice at 4°C for 4 h.
- For the ultrafiltration group, transfer the sample into a 10 kDa centrifugal filter unit, dilute to 500 μL with 1× PBS, and centrifuge at 10,000 × g, 4°C for 30 min. Concentrate the sample to approximately 100 μL, then spin at 8,000 × g for 2 min to collect. Aliquot 16 μL into a PCR tube (designated M2).
- Add 1 μL S1 (500 μM) to both the ultrafiltered and non-ultrafiltered samples and incubate on ice at 4°C for 4 h. Label the non-ultrafiltered sample N-U and the ultrafiltered sample U.
- Retain the −80°C storage aliquot as control C.
Native PAGE
- Gel: 7.5% native gel.
- Sample preparation: Mix 16 μL of each sample (C, M, N-U, U) with 4 μL loading buffer and mix thoroughly.
- Electrophoresis: Run at 120 V for 100 min in 1× TBE.

12.13
- S2 conjugated G5
Incubation
- Prepare two aliquots of G5 stock, labeled A and B.
- Add 1 µL DBCO to each of A and B and incubate for 4 h.
- For A: remove 16 µL as sample M1; add 10 µL S2 to the remaining A (named O1) and incubate for 4 h.
- For B: dilute the sample to 500 µL, transfer to a 10 kDa centrifugal ultrafiltration tube and centrifuge at 10,000 g for 30 min; concentrate to 100 µL, remove 16 µL as sample M2; add 10 µL S2 to the remaining B (named O2) and incubate for 4 h
Nativepage
- Gel:4% stacking gel / 10% resolving gel.
- Sample preparation: Mix 16 μL of each sample (C, A, M, B) with 4 μL loading buffer and mix thoroughly.
- Electrophoresis: Stacking Gel:75 V; Resolving Gel:100 V.1X TBE

12.15
- S2 conjugated enzyme
Sample preparation
- Bis-sulfone-PEG4-DBCO:dilute 2 µL DBCO in PBS (pH 7.5) to a final volume of 20 µL (1 mM). Incubate at 37 °C for 1 h to convert bis-sulfone to mono-sulfone (optimal pH ≈ 7.4).
- Enzyme:
- Prepare stocks of G5, Al, SS, RA, and RH (120 µL at 10 µM each). Dilute each stock to 240 µL with PBS (pH 7.0) to obtain 5 µM working solutions. (the optimal pH for the enzyme–DBCO reaction is 6.5–7.0.)
- Take 40 µL of each diluted G5, Al, SS, RA, and RH to use as parallel control samples.
Reaction
- Add 4 µL of the aqueous DBCO solution to each of G5, Al, SS, RA, and RH and incubate for 4 h. (Enzyme:DBCO ratio = 1:4.)
- Split each sample evenly into two aliquots: designate one as the M control, and subject the other to ultrafiltration and washing to remove excess reagents and ensure the final composition is unchanged. Incubate the samples on ice for 4 h.
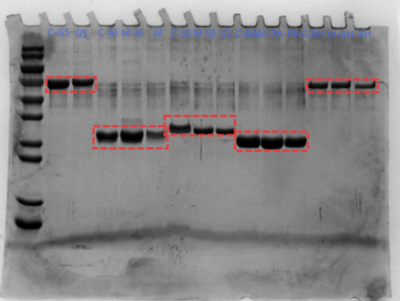
SDS page
- Gel:10% concentration
- Running Buffer:Tris:3.03g、Glycine:14.4g、SDS:1g、Water:1 L.
- Stain:Coomassie blue 37℃ Sway
- Sample:
- Mix 16 µL of sample with 4 µL loading buffer, vortex, and boil at 100 °C for 10 min to inactivate.
- Marker, C-G5, G5, C-Al, M-Al, Al, C-SS, M-SS, SS, C-RA, M-RA, RA, C-RH, M-RH, RH.
Result and Problem
- When DBCO (dissolved in DMF) is diluted into PBS, precipitation occurs.
12.17
- S2 conjugated enzyme
Sample preparation
- Bis-Sulfone-PEG4-DBCO;取DBCO存储液 10 ul,先加入60 ul DMF,再加入 30 ul 1X PBS(7.5),作为工作液(1 mM,30% 水)在 37℃孵育 1.5 h.
- Al、SS、RA:取Al、SS、RA 存储液,各用 1X PBS(pH 6.5)稀释至 240 ul(5 uM),各留样 30 ul 作为阴性对照,剩余作为工作液。
Reaction
| AL/ul | AL/ul | SS/ul | SS/ul | RA/ul | RA/ul | |
|---|---|---|---|---|---|---|
| Enzyme | 105 | 105 | 105 | 105 | 105 | 105 |
| DBCO | 5 (10:1) | 15 (30:1) | 10 (20:1) | 15 (30:1) | 5 (10:1) | 10 (20:1) |
| PBS (7.0) | 10 | 0 | 5 | 0 | 10 | 5 |
| Incubation / 5 h | on ice | on ice | on ice | on ice | on ice | on ice |
| SsDNA | S1 5 (10:1) | S1 15 (30:1) | S1 10 (20:1) | S2 15 (30:1) | S1 5 (10:1) | S1 10 (20:1) |
| Incubation / overnight | On ice | On ice | On ice | On ice | On ice | On ice |
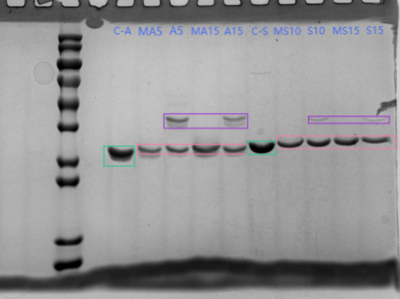
SDS page
- Gel:10% concentration
- Running Buffer:Tris:3.03g、Glycine:14.4g、SDS:1g、Water:1 L.
- Stain:Coomassie blue 37℃ Sway
- Sample:
- Mix 16 µL of sample with 4 µL loading buffer, vortex, and boil at 100 °C for 10 min to inactivate.
- Marker, C-A, M-A5, A5, M-A15, A15, C-S, M-S10, S10, M-S15, S15.
- Marker, C-R, M-R5, R5, M-R10, R10
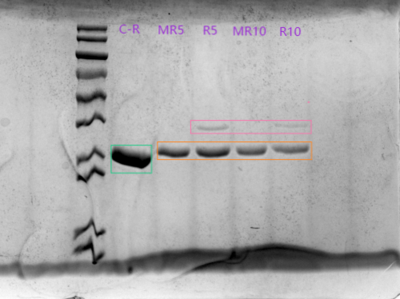
12.26
- Step one
- 取 2 ul DBCO 用 6 ul PBS(pH 7.5)和 12 ul DMF 稀释至 1 mM,37℃孵育1.5 h.
- 将 GDH、G5、Al、RH 放在冰上等待融化,留样 16 ul作为 C 组(C-GDH、C-G5、C-Al、C-RH);保证各样品为 116 ul 5uM.
- 向四个样品中各加入 5 ul 的孵育好的DBCO,在冰上 4 ℃孵育5 h.
- 制备Origami
- 混合二号位点3’处用S1’修饰的Staples(2-3'-1')--用来连接 S1-G5、S1-Al、S1-RH
- 混合一号位点3’处用S2’修饰的Staples(1-3'-2')--用来连接 S2-GDH
- 混合二号位点3’处 用S1’和 一号位点3’处用S2’修饰的Staples(2-3'-1'& 1-3'-2')--用来连接 S2-GDH & S1-G5; S2-GDH & S1-Al; S2-GDH & S1-RH.
- 制备Origami(NO)10 ul X 1;Origami(2-3'-1') 10 ul X 3;Origami(1-3'-2') 10 ul X 1;Origami(2-3'-1'& 1-3'-2') 10 ul X 3
- PCR 程序孵育
- Step two
- 分别留样孵育好的四个酶 16 ul,,作为 M 组(M-GDH、M-G5、M-Al、M-RH);向 GDH 样品中加入 5 ul S2,向 G5、Al、RH 样品中加入 5 ul S1,冰上 4 ℃孵育过夜
12.27
- step four
- 将孵育好的四个酶,取样 16 ul作为样品组(GDH、G5、Al、RH)
- 将 C、M、样品组跑SDS page(10%)
- Incubation
- 将四个酶样品用 1 X PBS(pH 7.0)洗3次
- 将酶和Origami混合 PCR孵育 4 h
| Origami (NO) | O-GDH | O-G5 | O-AI | O-RH | O-GDH-G5 | O-GDH-Almost | O-GDH-RH | |
|---|---|---|---|---|---|---|---|---|
| Origami/ul | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Enzyme/ul | 0 | 3 | 3 | 3 | 3 | 6 | 6 | 6 |
| PBS/ul | 6 | 3 | 3 | 3 | 3 | 0 | 0 | 0 |
- 琼脂糖凝胶电泳
