XIE/2:2026/Jan: Difference between revisions
(→1.28) |
|||
| (9 intermediate revisions by the same user not shown) | |||
| Line 25: | Line 25: | ||
===FRET=== | ===FRET=== | ||
*Method:Samples were measured in Cy3 and Cy5 channels using appropriate excitation/emission filters under constant instrument settings. | *Method:Samples were measured in Cy3 and Cy5 channels using appropriate excitation/emission filters under constant instrument settings. | ||
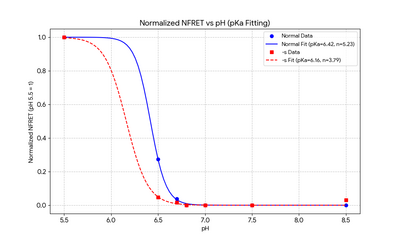
[[File:FRET-pH-260119.png|thumb|center|400px]] | |||
==1.20== | ==1.20== | ||
===Antibody reduction=== | ===Antibody reduction=== | ||
| Line 37: | Line 39: | ||
===Native-page=== | ===Native-page=== | ||
*Analyze reaction products by non‑denaturing electrophoresis using 7.5% native PAGE. | *Analyze reaction products by non‑denaturing electrophoresis using 7.5% native PAGE. | ||
*Marker、Control(antibody)、Antibody-Oligo、Antibody-DNA、Marker | |||
[[File:Ab-Oligo Native 7.5 2026-01-23.jpg|thumb|center|400px]] | [[File:Ab-Oligo Native 7.5 2026-01-23.jpg|thumb|center|400px]] | ||
Conclusion :Nativepage is not a good chose antibody conjugation | Conclusion :Nativepage is not a good chose antibody conjugation | ||
| Line 42: | Line 45: | ||
==1.21== | ==1.21== | ||
===Native-page=== | ===Native-page=== | ||
Samples(1.20 antibody with oligo) and controls were analyzed by native polyacrylamide gel electrophoresis (PAGE) using a 3–5% stacking gel and a 12.5% resolving gel. | *Samples(1.20 antibody with oligo) and controls were analyzed by native polyacrylamide gel electrophoresis (PAGE) using a 3–5% stacking gel and a 12.5% resolving gel. | ||
*Marker、Control(antibody)、Antibody-Oligo、Antibody-DNA、Marker | |||
[[File:Ab-Oligo_Native4-12.5_2026-01-23.jpg|thumb|center|400px]] | |||
==1.22== | |||
==1.28== | |||
===抗体偶联=== | |||
===平均偶联数量测量=== | |||
*双波长法计算标记度(Degree of Labeling, DoL) | |||
以下公式采用 A280 和 A260 的吸光度值,通过校正 DNA 在 280 nm 处的贡献来计算抗体浓度、核酸浓度及最终的平均偶联数(DoL)。 | |||
#CAb = [A280 − CF_DNA,280·A260] / εAb,280·L | |||
#CDNA = A260 / εDNA,260·L | |||
#DoL = CDNA / CAb、 | |||
*L 光程 这里为 1;A280,A260 样品吸光度; | |||
*CF_DNA,280·A260 “纯DNA”在同缓冲下测得的比值 A280/A260,用来估算DNA在280的交叉吸收; | |||
*εAb,280 抗体消光系数,本抗体为2.3865×10^5 M⁻¹·cm⁻¹; | |||
*εDNA,260 Oligo消光系数,该Oligo(TTTTCTCCTCTCTCCTCCTCTT,5'Azide)为 1.698×10^5 M⁻¹·cm⁻¹; | |||
===SDS-page验证偶联结果=== | |||
Latest revision as of 12:55, 28 January 2026
1.13[edit]
Synthesis of Anti-Oligo
抗体还原[edit]
- 取2 ul(1mM) TCEP,用 PBS(7.4) 稀释至 20 ul(100uM),取 5 ul稀释后的 TCEP ,加入 85 ul PBS(7.40),涡旋混匀---TCEP溶液pH过低提前中和
- 取2 ul(50uM)抗体,用 PBS(pH 7.4)稀释至 10 ul(10uM),将 TECP 的 PBS 缓冲液加入其中,37℃ 孵育 2 h.
- 取1 ul(50uM)抗体,用 PBS(pH7.4)稀释至 8 ul,作为 C 组
偶联[edit]
- 将孵育好的溶液用 PBS(6.5) 洗涤4次(10000g 3.5min),均分样品(A.B)----buffer pH 6.5避免后续加入的Mal被水解(碱性易水解)
- A(共混组):向 A 中加入 5 ul(100uM) 的 Mal-PEg-DBCO 和 1 ul(1mM) Azide-ssDNA,室温避光孵育 4 h.
- B(依次组):1)向 B 中加入 5 ul(100uM) 的 Mal-PEg-DBCO,室温避光孵育 2 h. 2)孵育结束后,向其加入1 ul(1mM) Azide-ssDNA,室温避光孵育 2 h.
验证[edit]
- 强还原断开轻重链:向A、B、C中加入对应量的蛋白 loudingbuffer ,再各加入1 ulβ-巯基乙醇,100℃煮 10m in
- 10% SDS-Page.
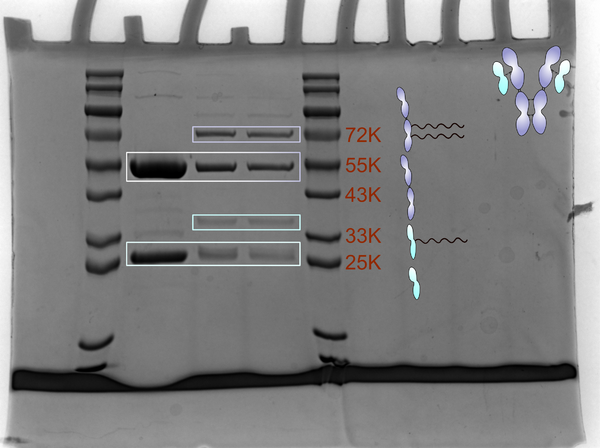
1.19[edit]
incubation[edit]
- DNA samples were stratified by thiolation status into two primary groups: thiolated DNA (bearing a thiol modification) and non-thiolated DNA (without thiol modification). Within each primary group, samples were further subdivided by phosphate-buffered saline (PBS) pH, yielding subgroups at pH 5.5, 6.5, 6.7, 6.8, 7.0, 7.5, and 8.5. PBS solutions were prepared and adjusted to the target pH and verified with a calibrated pH meter at room temperature.
| S1 (100 μM) | S2 (100 μM) | TFO (100 μM) | Buffer |
|---|---|---|---|
| 2 μl | 2 μl | 2 μl | 194 μl |
FRET[edit]
- Method:Samples were measured in Cy3 and Cy5 channels using appropriate excitation/emission filters under constant instrument settings.
1.20[edit]
Antibody reduction[edit]
- Dilute 2 µL of antibody stock with 1× PBS to 10 µL (10 µM).
- Prepare a TCEP working solution by diluting 1.5 µL of 100 µM TCEP with 1× PBS to 90 µL.
- Mix the 10 µL antibody with 90 µL TCEP working solution and incubate at 37°C for 2.5 h (final antibody ≈1 µM; TCEP ≈1.5 molar equivalents).
- Purify the reduced antibody by three rounds of buffer exchange into 1× PBS.
Antibody functionalization with DBCO[edit]
- Add 5 µL of Mal‑PEG4‑DBCO (100 µM) to the purified, reduced antibody and incubate at 37°C for 2 h (5 eq).
Antibody conjugation to oligo[edit]
- Add 5 µL of Azide‑Oligo (100 µM) to the DBCO‑modified antibody and incubate at 37°C for 2 h to complete the strain‑promoted azide‑alkyne cycloaddition (SPAAC) coupling (5 eq).
Native-page[edit]
- Analyze reaction products by non‑denaturing electrophoresis using 7.5% native PAGE.
- Marker、Control(antibody)、Antibody-Oligo、Antibody-DNA、Marker

Conclusion :Nativepage is not a good chose antibody conjugation
1.21[edit]
Native-page[edit]
- Samples(1.20 antibody with oligo) and controls were analyzed by native polyacrylamide gel electrophoresis (PAGE) using a 3–5% stacking gel and a 12.5% resolving gel.
- Marker、Control(antibody)、Antibody-Oligo、Antibody-DNA、Marker
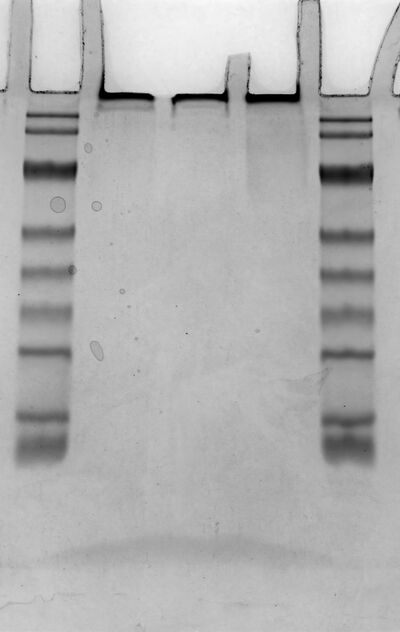
1.22[edit]
1.28[edit]
抗体偶联[edit]
平均偶联数量测量[edit]
- 双波长法计算标记度(Degree of Labeling, DoL)
以下公式采用 A280 和 A260 的吸光度值,通过校正 DNA 在 280 nm 处的贡献来计算抗体浓度、核酸浓度及最终的平均偶联数(DoL)。
- CAb = [A280 − CF_DNA,280·A260] / εAb,280·L
- CDNA = A260 / εDNA,260·L
- DoL = CDNA / CAb、
- L 光程 这里为 1;A280,A260 样品吸光度;
- CF_DNA,280·A260 “纯DNA”在同缓冲下测得的比值 A280/A260,用来估算DNA在280的交叉吸收;
- εAb,280 抗体消光系数,本抗体为2.3865×10^5 M⁻¹·cm⁻¹;
- εDNA,260 Oligo消光系数,该Oligo(TTTTCTCCTCTCTCCTCCTCTT,5'Azide)为 1.698×10^5 M⁻¹·cm⁻¹;